Hydroxide Precipitation of Metals

Hydroxide Precipitation of Metals
The most common used method to remove soluble metal ions from solution is to precipitate the ion as a metal hydroxide. The process is readily automated and controlled by a simple pH controller. By raising the pH value of a solution with a common alkaline material such as lime, or sodium hydroxide the corresponding metallic hydroxide compounds become insoluble and precipitate from solution.
Below is a metal hydroxide solubility curve showing the solubility of the common heavy metal ions and their respective solubility versus pH.
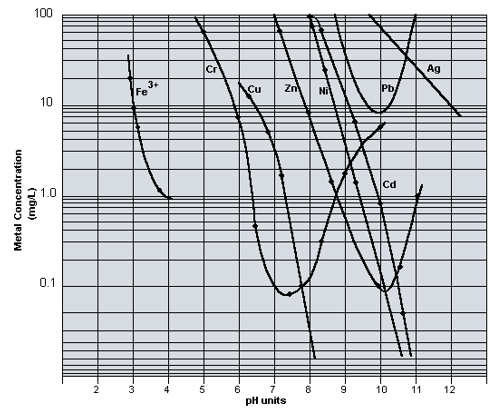
- If copper is reviewed, it is seen that at a pH of 6 copper has a solubility of 20 mg/l and at a pH of 8.0, the solubility is 0.05 mg/l.
- Nickel has a similar curve but it occurs at 3 pH points high. At a pH of 8.0 nickel has a solubility of 70 mg/l and at a pH of 10.2 the solubility is 0.1 mg/l.
- Several metals such as chromium and zinc are amphoteric, being soluble at both alkaline and acid conditions. Chromium reaches its least theoretical chromium solubility of 0.08 at pH of 7.5.
- If both chromium and nickel are present a pH value that precipitates both ions must be chosen. It is common to utilize a pH of 9.0 – 9.5 to precipitate both metals.
- The theoretical solubility usually does not exist in practice. Metallic coagulant such as ferric chloride or aluminum sulfate are generally used to accelerate the coagulation and precipitation of the heavy metals. Even when not added they are present from other metal processing solutions such as the pickling bath. Ferric hydroxide and/or aluminum hydroxide precipitate and tend to form co-precipitate with nickel and chromium. The net is a metallic ion concentration lower than would be predicted from the solubility curve.
- The effluent limitations for chromium and nickel are both 2.4 mg/l to discharge to a city sewer in the U.S. A pH value of 9 – 9.5 will usually precipitate both ions to their required level.
- If chromium must be precipitated to a level less than 0.5 mg/l the pH must be operated at 7.0-8.0. If nickel is present it must be precipitated with sulfide as the metallic sulfide ion. Chromium does not form insoluble sulfide precipitates and must be precipitated as the hydroxide at 7.0 – 8.0.
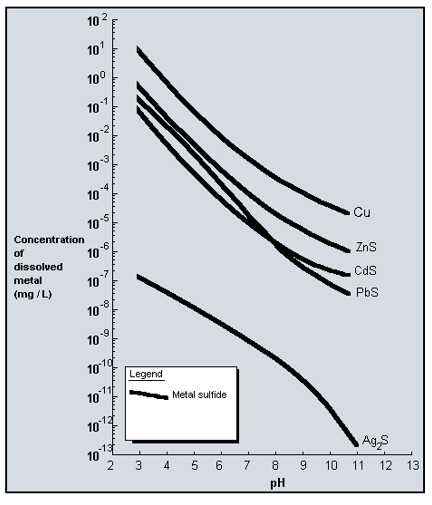
- Attached is the heavy metal sulfide solubility curves. The sulfide solubility is several orders of magnitude lower than the comparable hydroxide.
- Ammonical Complexes
- Most heavy metal ions readily precipitate by raising the pH of solution, forming the respective metal hydroxide compound. A hydroxide precipitation curve is attached demonstrating the relationship
- Certain metal ions, primarily copper, zinc and cadmium readily form metallic complexes with ammonia. The ammonical metal complexes remain vary soluble at the higher pH values prohibiting the precipitation of the respective metal hydroxide. There are several methods conventionally used to destroy the ammonical complex and precipitate the metallic ion.
- The ammonia ion may be destroyed by oxidation with chlorine or ozone. Eliminating the ammonia destroys the complex. However, the cost is prohibitive when compared to other methods.
- The addition of soluble ferrous ion as either ferrous sulfate or ferrous chloride will co-precipitate the metallic ion with the iron hydroxide. The most economical method is to add soluble sulfide ions and break the ammonical complex by precipitating the metallic sulfide compounds. The sulfide solubility chart below demonstrates the solubility of the metal sulfide compounds. Copper sulfide, for example, is a very insoluble compound and the presences of soluble sulfide precipitates the copper as it dissociates from the ammonical complex. Ultimately, the copper is all removed from the complex and precipitated as copper sulfide. The ammonia remains in the solution.
= = = = = = = = = = = = = = = = = = = = = = = = = = = = = =
The most economical method is to add soluble sulfide ions and break the ammonical complex by precipitating the metallic sulfide compounds. The sulfide solubility chart below demonstrates the solubility of the metal sulfide compounds. Copper sulfide, for example, is a very insoluble compound and the presences of soluble sulfide precipitates the copper as it dissociates from the ammonical complex. Ultimately, the copper is all removed from the complex and precipitated as copper sulfide. The ammonia remains in the solution.