Cyanide Oxidation

Cyanide Oxidation
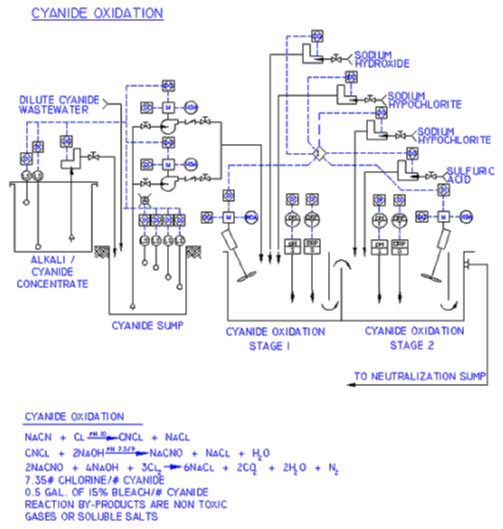
Alkaline cyanide solutions are very common for the electrodepositions of cadmium, copper, zinc, brass, silver, and gold. These toxic metal wastewaters require destruction of the cyanide before chemical neutralization. Sodium hypochlorite, a 15% solution of chlorine gas in sodium hydroxide solution, oxidizes the cyanide to a less toxic cyanate while sodium hydroxide (caustic) controls the levels of pH. The chemical equation below represents the reaction.
![]()
A gold ORP electrode and ORP controller/meter automatically call for additional sodium hypochlorite (chlorine) whenever the ORP potential falls below +200MV.
Stoichiometrically, the oxidation of cyanide requires seven (7) pounds of chlorine per one (1) pound of cyanide. The metering pumps have sufficient capacity to deliver the required volume.
A pH electrode and controller/meter automatically call for additional caustic whenever the pH falls below 10.5. Cyanogen chloride (a lachrymator) initially is formed and subsequently oxidized to a cyanate. Cyanogen chloride is soluble in water at a pH above 10. pH is maintained at 10.5 or above to retain the cyanogen chloride in solution.
Sodium hypochlorite and sodium hydroxide are mixed with the wastewater via a ¼ horsepower mixer to assure rapid oxidation of the cyanide to cyanate and to prevent the release of cyanogen chloride. The reaction tank is equipped with baffles for increased mixing and to prohibit “vortexing.” The cyanide oxidation system is also equipped with a “stand alone” control panel. The panel is composed of both pH and ORP controller/meters which display current oxidation and pH levels and automatically adjusts for any type of out-of-balance conditions.
In the event an excessive cyanide concentration enters the oxidation chamber, or the reagent chemical delivery system fails causing a temporary depletion in the chlorine oxidant, an inadequate treatment condition will occur. This is indicated by an amber warning light on the graphic display of the control panel. Mixing continues and reagents are supplied to the reaction zone until the ORP and/or pH values return to the normal operating range.